Novel human lung organoids to study development and disease
December 8, 2022
Read more
This work helps us to understand how asymmetry in a female germline cyst is converted into a polarised microtubule network that is essential for delivery of oocyte fate determinants to the future oocyte.
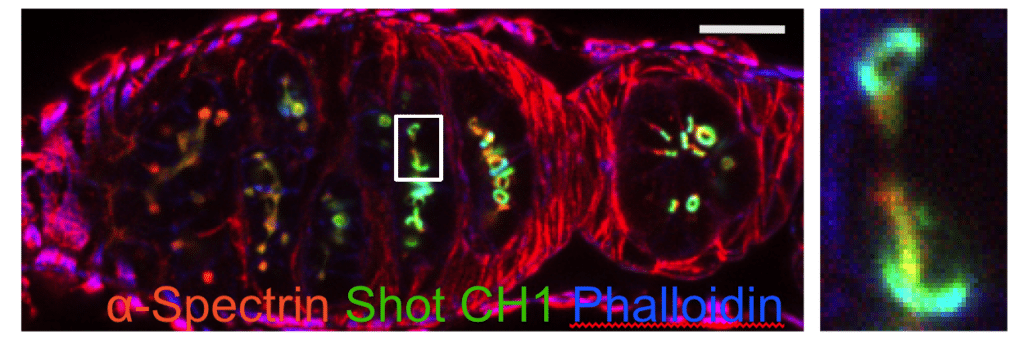
Drosophila germarium with germline cysts expressing calponin homoly domain from Spectraplakin Shot (green), stained with a-Spectrin antibody (red) and Phalloidin (blue). The righthand panel shows the enlargement of the boxed region in the lefthand panel.
In Drosophila, only one cell in a multicellular female germline cyst is specified as an oocyte and a similar process occurs in mammals. The symmetry-breaking cue for oocyte selection is provided by the fusome, a tubular structure connecting all cells in the cyst. The Drosophila spectraplakin Shot localises to the fusome and translates its asymmetry into a polarised microtubule network that is essential for oocyte specification, but how Shot recognises the fusome is unclear. Here, we demonstrate that the actin-binding domain (ABD) of Shot is necessary and sufficient to localise Shot to the fusome and mediates Shot function in oocyte specification together with the microtubule-binding domains. The calponin homology domain 1 of the Shot ABD recognises fusomal F-actin and requires calponin homology domain 2 to distinguish it from other forms of F-actin in the cyst. By contrast, the ABDs of utrophin, Fimbrin, Filamin, Lifeact and F-tractin do not recognise fusomal F-actin. We therefore propose that Shot propagates fusome asymmetry by recognising a specific conformational state of F-actin on the fusome.