NanoDam finds missing temporal factors
May 9, 2022
Read more
Oocyte identity is established inside a cyst of interconnected germ cells but how this occurs is not well understood. Using Drosophila as a model, the St Johnston lab reveal a molecular mechanism underlying the oocyte specification process. Initial asymmetry in the cyst results from unequal divisions during cyst formation. The microtubule minus end-binding protein Patronin/CAMSAP translates the asymmetry into a polarised microtubule network that directs transport of oocyte fate determinants into a single germ cell to establish the oocyte.
Nashchekin D et al. (2021) Symmetry breaking in the female germline cyst. Science 374: 874-879. DOI: 10.1126/science.abj3125
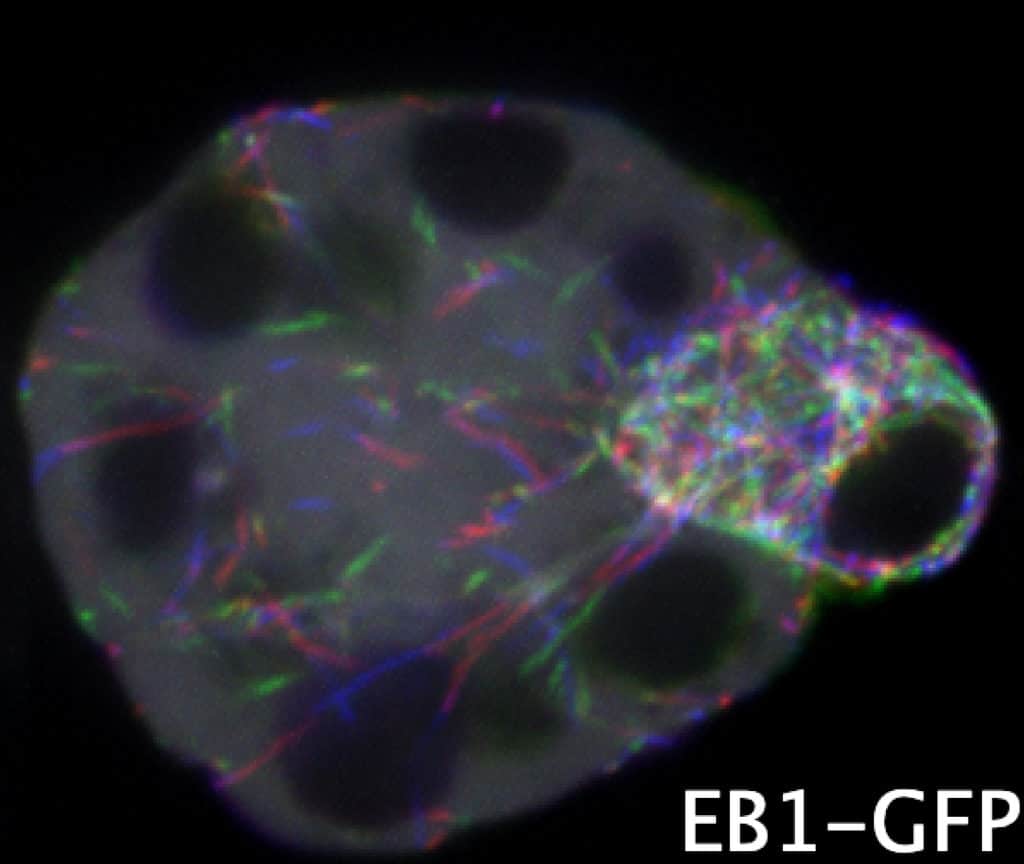
In mammals and flies, only one cell in a multicellular female germline cyst becomes an oocyte, but how symmetry is broken to select the oocyte is unknown.
Here, we show that the microtubule (MT) minus end-stabilizing protein Patronin/CAMSAP marks the future Drosophila oocyte and is required for oocyte specification. The spectraplakin Shot recruits Patronin to the fusome, a branched structure extending into all cyst cells. Patronin stabilizes more MTs in the cells with the most fusome material.
Our data suggest that this weak asymmetry is amplified by Dynein-dependent transport of Patronin-stabilized MTs. This forms a polarized MT network, along which Dynein transports oocyte determinants into the presumptive oocyte. Thus, Patronin amplifies a weak fusome anisotropy to break symmetry and select one cell to become the oocyte.