Jenny Gallop
Group leaderResearch summary
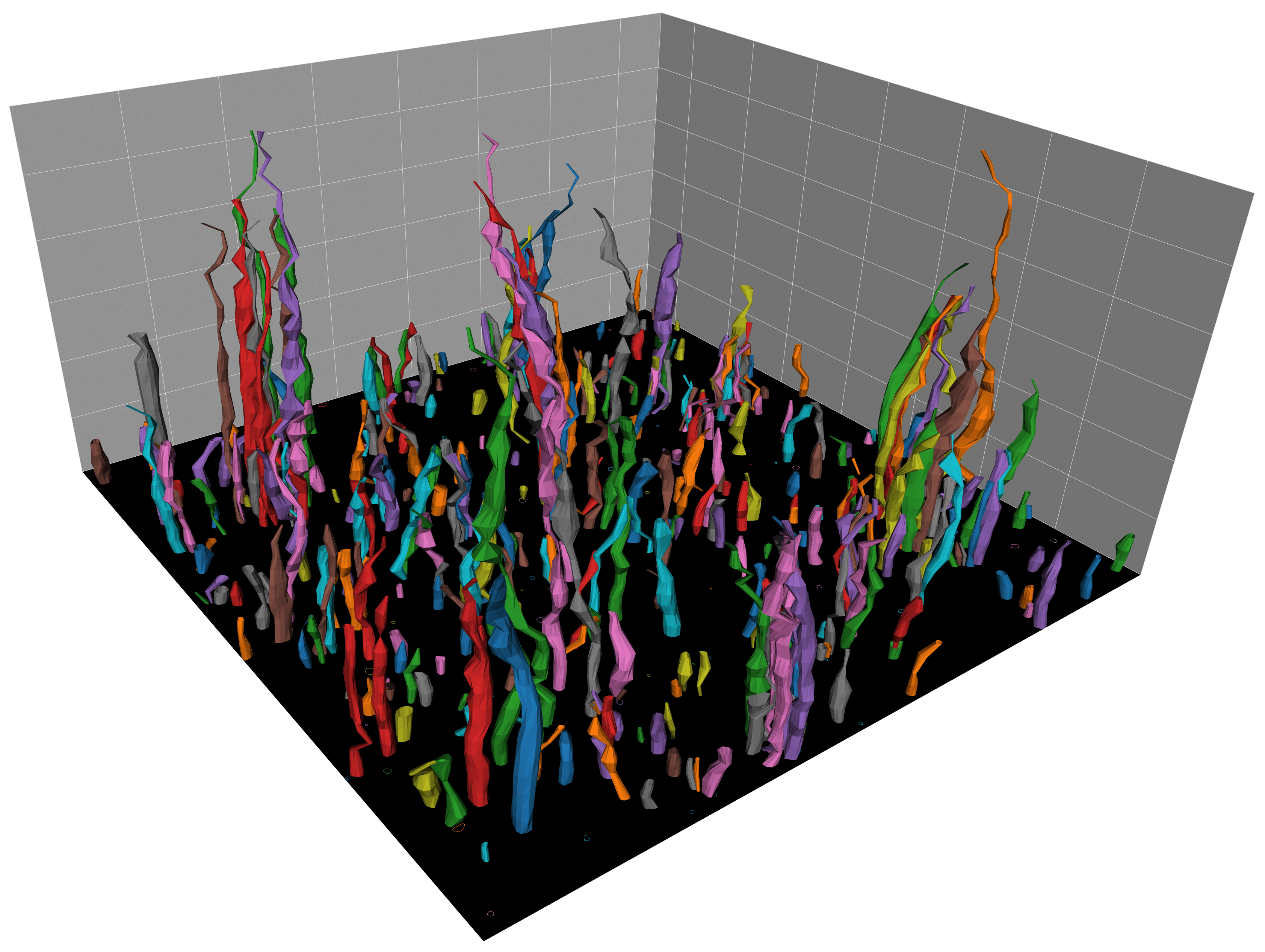
Signalling to the actin cytoskeleton
Cells move during embryonic development and throughout the life of an organism. When they move, cells reorganise a system of filaments - the actin cytoskeleton - that gives them their shape and exerts force on the surrounding tissues. When regulation of the actin cytoskeleton is disrupted it can lead to cancer metastasis, intellectual disability, kidney dysfunction and other problems.

Selected publications
-
Blake TCA and Gallop JL. (2023) Filopodia in vitro and in vivo. Annu Rev Cell Dev Biol Online ahead of print DOI: 10.1146/annurev-cellbio-020223-025210.
-
Dobramysl U et al. (2021) Stochastic combinations of actin regulatory proteins are sufficient to drive filopodia formation. J Cell Biol 220: e202003052. DOI: 10.1083/jcb.202003052.
-
Berquez M et al. (2020) The phosphoinositide 3-kinase inhibitor alpelisib restores actin organization and improves proximal tubule dysfunction in vitro and in a mouse model of Lowe syndrome and Dent disease. Kidney Int. 98: 883–896. DOI: 10.1016/j.kint.2020.05.040.
-
Jarsch IK et al. (2020) A role for SNX9 in the biogenesis of filopodia. J Cell Biol 219(4): e201909178. DOI: 10.1083/jcb.201909178.
-
Daste F et al. (2017) Control of actin polymerization via the coincidence of phosphoinositides and high membrane curvature. J Cell Biol 216: 3745–3765. DOI: 10.1083/jcb.201704061.
Biography
Dr Jenny Gallop PhD, Group Leader, Wellcome Trust Senior Research Fellow, Associate Professor in the University Department of Biochemistry
Jenny grew up in Bristol, UK and studied Molecular and Cellular Biochemistry at Trinity College, University of Oxford where she was awarded the Titley Exhibition and graduated with First Class Honours in 2001. She gained a Medical Research Council Pre-doctoral Fellowship to undertake her PhD at the Laboratory of Molecular Biology in Cambridge, where she discovered how BAR domain proteins generate membrane curvature with Dr Harvey McMahon and Dr Phil Evans, for which she was awarded the Max Perutz student prize.
Gaining an EMBO fellowship in 2006, Jenny moved field towards cell biology and development, starting her work on the actin cytoskeleton using Xenopus as a model system in her postdoc with Professor Marc Kirschner at Harvard Medical School. Dr Gallop returned to the UK in 2011 to set up her lab at the Gurdon Institute with a Wellcome Trust Career Development Fellowship and an ERC Starting Grant in 2012.
Her work is highly interdisciplinary, incorporating biochemical mechanism, advanced in vivo and in vitro imaging, quantification and disease models to understand the mechanisms of filopodia formation and actin regulation by phosphoinositide lipids. She has translated her discoveries into rare disease and was appointed to the Medical and Scientific Advisory boards of the Lowe Syndrome Association and Dent Disease Foundation in 2021 in recognition of her contributions to mechanistic understanding and drug repurposing.
Notable achievements and honours
-
2020Associate Professor, Department of Biochemistry
-
2020Wellcome Trust Senior Research Fellowship
-
2015AcademiaNet Selection
-
2012ERC Starting Grant
-
2011Wellcome Trust Research Career Development Fellowship
-
2006EMBO Long term Fellowship
-
2005Max Perutz student Prize
-
2001MRC Predoctoral Fellowship
Research group
-
Dr Thomas Blake
Research Associate
-
Dr Jonathan Gadsby
Research Associate
-
Pia Jeyarajasingham
PhD Student
-
Julia Mason
Research Associate
-
Alex Sutherland
MPhil Student
-
Pankti Vaishnav
PhD Student/ Research Assistant