Fengzhu Xiong
Group leaderResearch summary
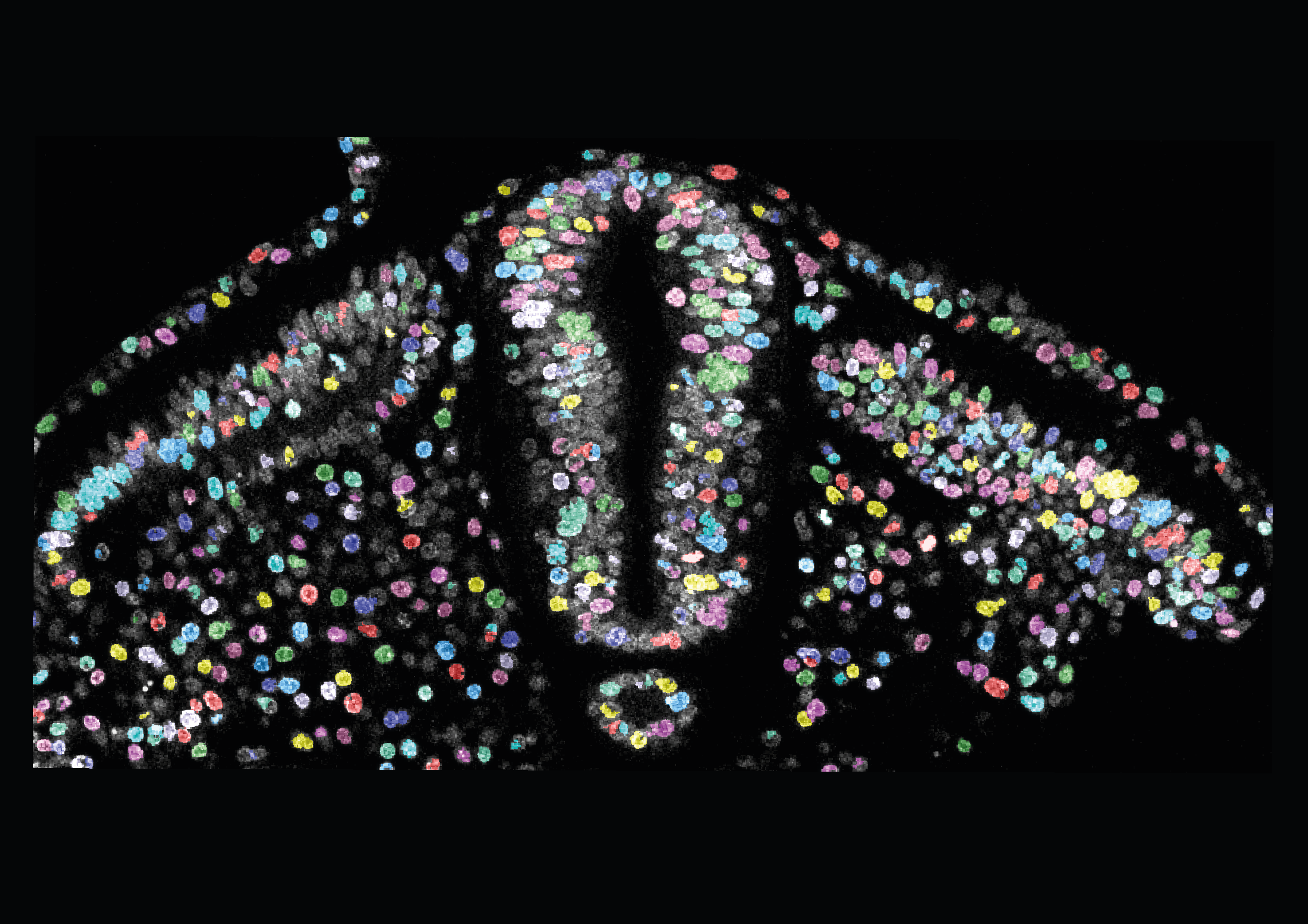
Tissue morphogenesis by mechanics and cell dynamics
What forces drive tissue morphogenesis? Embryos are made of soft materials consisting of cells with limited mechanical capacities, yet they develop in a robust and coordinated manner and produce large-scale deformations (morphogenesis). We are interested in the ways in which developing tissues produce and respond to mechanical forces in order to achieve the correct shape and pattern.
Selected publications
-
McLaren SBP, Xue S, Ding S, Winkel A, Baldwin O, Dwarakacherla S, Franze K, Hannezo E, Xiong F. (2024) Differential tissue deformability underlies shape divergence of the embryonic brain and spinal cord under fluid pressure. BioRxiv. DOI: 10.1101/2024.01.12.575349.
-
Lu C* Vidigueira JMN*, Chan JJC*, Maksymiuk A, Xiong F. (2024) Cell density couples tissue mechanics to control the elongation speed of the body axis”, (*equal contribution) BioRxiv. DOI: 10.1101/2023.12.31.573670.
-
Kunz D et al. (2023) Downregulation of extraembryonic tension controls body axis formation in avian embryos. Nat Commun 14, 3266. DOI: 10.1038/s41467-023-38988-3.
-
Chan CU et al. (2023) Direct force measurement and loading on developing tissues in intact avian embryos. Development 150(9):dev201054. DOI: 0.1242/dev.201054.
-
Moon LD & Xiong F (2021) Mechanics of neural tube morphogenesis. Seminars in Cell & Developmental Biology. DOI: 10.1016/j.semcdb.2021.09.009.
-
Xiong F et al. (2020) Mechanical Coupling Coordinates the Co-elongation of Axial and Paraxial Tissues in Avian Embryos. Developmental Cell 55:354–366.e5. DOI: 10.1016/j.devcel.2020.08.007.
Biography
Fengzhu Xiong PhD
Wellcome Sir Henry Dale Fellow, Wellcome-Beit Prize Fellow, Member of the University Department of Physiology, Development and Neuroscience
Fengzhu earned his BS degree at Tsinghua University and his PhD degree at Harvard University. His PhD dissertation work with Prof Sean Megason focused on imaging and modelling of cell dynamics to understand pattern formation and morphogenesis during development in zebrafish embryos. He discovered a cell sorting process for neural progenitor domains and an interplay between cell shapes and divisions for robust epithelial morphology. Fengzhu then conducted his postdoctoral training with Profs Olivier Pourquié and L. Mahadevan at Harvard, shifting focus to tissue mechanics. He characterized the “elongation engine” that utilizes inter-tissue mechanical interactions to coordinate the growth of multiple tissues during body axis formation in chicken embryos, leading to the focus on systems tissue mechanics in his group at the Gurdon Institute.
Fengzhu’s research group at the Gurdon Institute now combine expertise from biology, physics and engineering to develop novel tools and models to measure forces and mechanical properties of tissues during morphogenesis, to uncover how developmental mechanisms navigate and take advantage of the physical constraints. The lab has developed tension and pressure modulators, control-based cantilever actuator systems (Tissue force microscopy, TiFM), and is working on magnetic micro/nanorobotic approaches. The biological questions of interest include the maintenance of body axis symmetry during elongation and the mechanical regulation of neural tube morphogenesis, among many others.
Recent works by lab members have characterized a role of extraembryonic tension on body axis morphogenesis (Kunz et al.), a role of cell density in regulating elongation speed (Lu et al.), and a tissue mechanical property pattern in prescribing the expanded shape of the brain in contrast to the spinal cord (McLaren et al.). The group also collaborate widely. Examples include working with theoreticians to create morphogenesis models using mechanical measurements made in the lab, providing chicken embryos as a testing model for novel tools developed by bioengineers, and applying novel mechanical tools to help test hypotheses in other systems generated by biologists.
Notable achievements and honours
-
2022-2027UKRI Horizon / ERC Starting Grant
-
2019-2024Sir Henry Dale Fellowship
-
2019Wellcome-Beit Prize
-
2018-2022Pathway to Independence Award, NIH
-
2015-2017Helen Hay Whitney Fellowship
Research group
-
Dr Lakshmi Balasubramaniam
Research Associate
-
Yixin Dai
PhD Student
-
Ana Hernandez Rodrigues
PhD Student
-
Emily Holmes
Research Assistant
-
Dr Fengtong Ji
Research Associate
-
Yisha Lan
PhD Student
-
Dr Susie McLaren
Research Associate
-
Lauren Moon
PhD Student
-
Joana Vidigueira
Research Assistant and Lab Manager