Sumru Bayin
Group leaderResearch summary
Molecular mechanisms that regulate stem cell behaviours and age-dependent regenerative mechanisms in the brain
Our lab is interested in answering two overarching questions:
1. What are the cellular and molecular mechanisms that enable regeneration in the neonates and inhibit in the adult?
2. Can we facilitate regeneration in the brain?
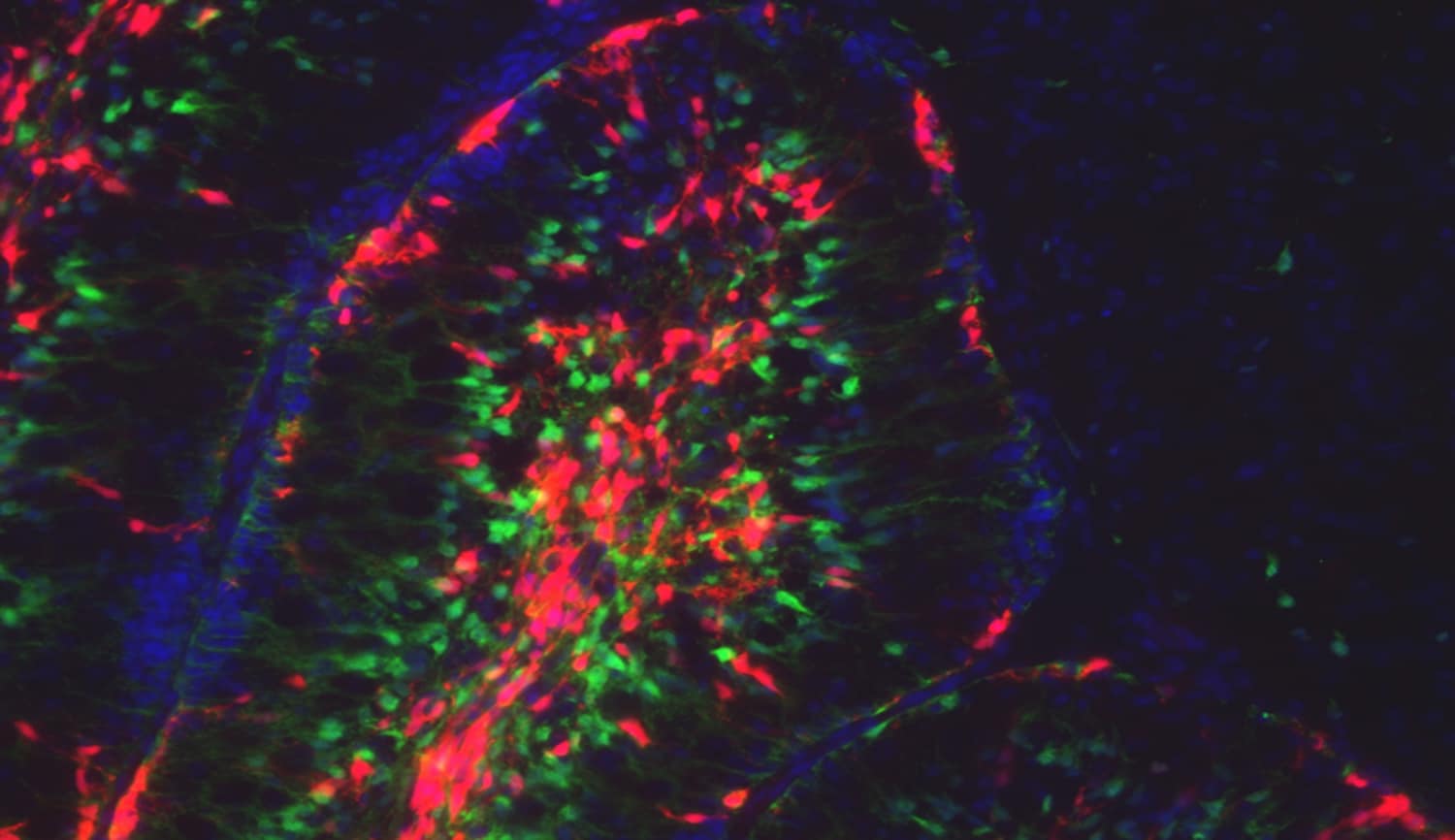
Using neonatal cerebellum as a paradigm, our goal is to answer fundamental questions about neural stem cells and their behaviours using:
1. Genetically-engineered mouse models
2. Single cell genomics
3. In vivo injury models and in vitro stem cell assays

Selected publications
-
Bayin NS et al. (2021) Injury induced ASCL1 expression orchestrates a transitory cell state required for repair of the neonatal cerebellum. Science Adv. 7(50). DOI: 10.1126/sciadv.abj1598.
-
Bayin NS et al. (2018) Age-dependent dormant resident progenitors are stimulated by injury to regenerate Purkinje neurons. eLife 7:e39879. DOI: 10.7554/eLife.39879.
-
Wojcinski A et al. (2017) Cerebellar Granule Cell Replenishment Post-Injury by Adaptive Reprogramming of Nestin+ Progenitors. Nature Neuroscience 20: 1361–1370. DOI:10.1038/nn.4621.
-
Bayin NS et al. (2017) Notch signaling regulates metabolic heterogeneity in glioblastoma stem cells. Oncotarget 8:64932-64953. DOI:10.18632/oncotarget.18117.
-
Bayin NS et al. (2016) GPR133 (ADGRD1), an adhesion G protein-coupled receptor, is necessary for glioblastoma growth. Oncogenesis 5, e263 DOI:10.1038/oncsis.2016.63.
Biography
Sumru is a stem cell biologist with a long-standing interest in studying stem cell diversity and molecular regulation of stem cell behaviours in the brain.
She completed her BSc and MSc in Molecular Biology and Genetics at Bilkent University (Ankara, Turkey) and her PhD in Stem Cell Biology at NYU Langone School of Medicine (New York, USA) in the laboratory of Dr Dimitris Placantonakis. Sumru completed her postdoctoral training at Memorial Sloan Kettering Cancer Centre (New York, USA) in the laboratory Dr Alexandra Joyner.
Sumru has received a number of awards including NIH Pathway to Independence Award (K99/R00), Sloan Kettering Institute Postdoctoral Research Award and was selected as a 2020 Leading Edge Fellow.
Notable achievements and honours
-
2023Wellcome Career Development Award
-
2020Leading Edge Fellow
-
2018NIH Pathway to Independence Award
Research group
-
Dr James Bae
Research Associate
-
Amanda Chan
MPhil Student
-
Jens Bager Christensen
PhD Student
-
Niamh Divers
PhD Rotation Student
-
Elena Moradi
Research Assistant
-
Ryan Smith
Part II Student
-
Giada Vanacore
PhD Student