Steve Jackson
Associate group leaderResearch summary
Maintenance of genome stability
DNA is constantly damaged by environmental and endogenously arising agents. Cell survival and genome integrity are promoted by DNA repair and associated processes, collectively known as the DNA-damage response (DDR). DDR defects are associated with developmental disorders, immunodeficiencies, infertility, premature ageing and cancer.
Our research aims to characterise the cell biology and mechanisms of established and new DDR components and pathways, and to identify ways to translate this knowledge to better understand and treat human diseases.

Selected publications
-
D’Alessandro G et al. (2023) RAD54L2 counters TOP2-DNA adducts to promote genome stability. Science Advances 9(49):eadl2108. DOI: 10.1126/sciadv.adl2108.
-
Serrano-Benitez A et al. (2023) Unrepaired base excision repair intermediates in template DNA strands trigger replication fork collapse and PARP inhibitor sensitivity. EMBO Journal 42(18):e113190. DOI: 10.15252/embj.2022113190.
-
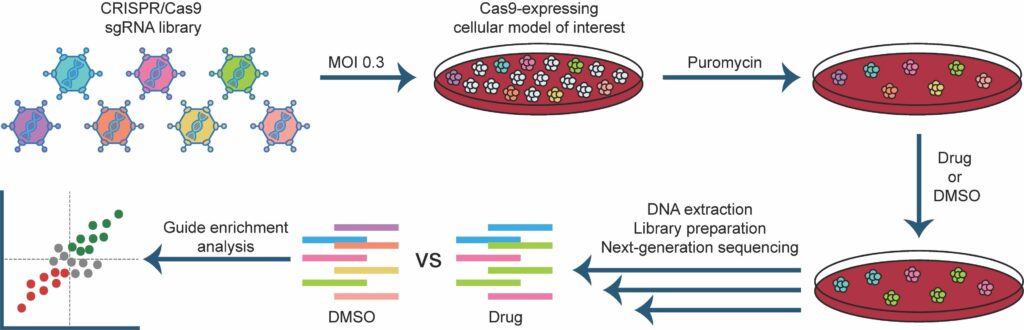
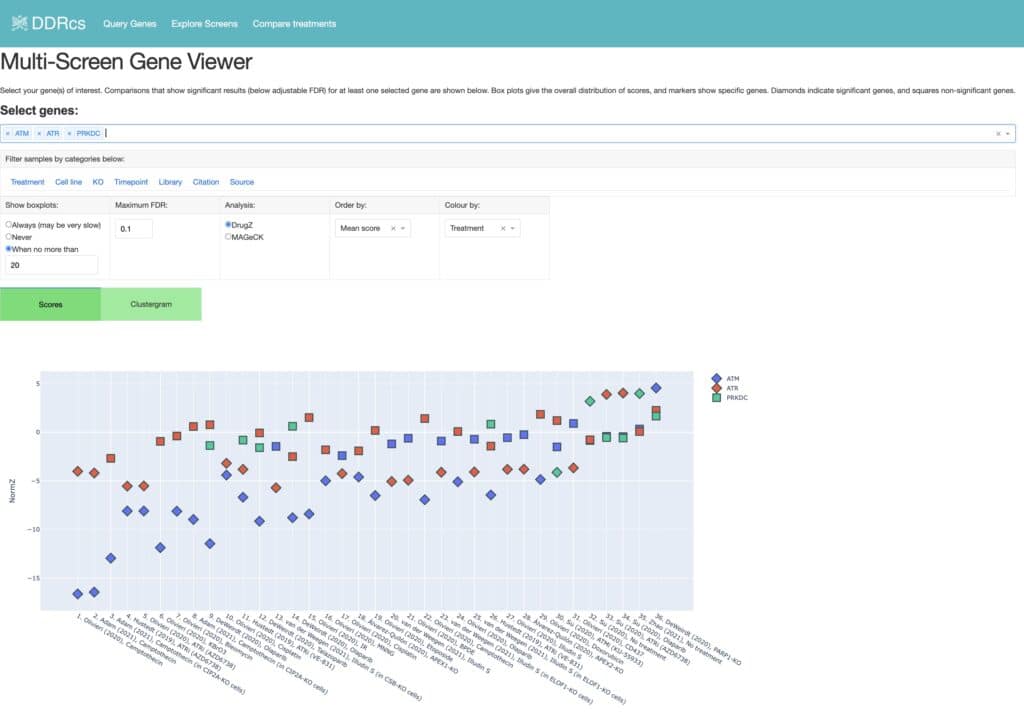
Awwad SW et al. (2023) Revolutionizing DNA repair research and cancer therapy with CRISPR-Cas screens. Nat Rev Mol Cell Biol 24(7):477-494. DOI: 10.1038/s41580-022-00571-x.
-
Herzog M et al. (2021) Mutagenic mechanisms of cancer-associated DNA polymerase ϵ alleles. Nucleic Acids Research 49(7): 3919-3931. DOI: 10.1093/nar/gkab160.
-
Lloyd RL et al. (2021) Loss of Cyclin C or CDK8 provides ATR inhibitor resistance by suppressing transcription-associated replication stress. Nucleic Acids Res 49(15):8665-8683. DOI 10.1093/nar/gkab628.
-
Bowden AR et al. (2020) Parallel CRISPR-Cas9 screens clarify impacts of p53 on screen performance. eLife 9:e55325. DOI: 10.7554/eLife.55325.
-
Belotserkovskaya R et al. (2020) PALB2 chromatin recruitment restores homologous recombination in BRCA1-deficient cells depleted of 53BP1. Nature Communications 11: 819. DOI: 10.1038/s41467-020-14563-y.
-
Salguero I et al. (2019) MDC1 PST-repeat region promotes histone H2AX- independent chromatin association and DNA damage tolerance. Nature Communications 10: 5191. DOI: 10.1038/s41467-019-12929-5.
-
Puddu F et al. (2019) Genome architecture and stability in the Saccharomyces cerevisiae knockout collection. Nature 537:416-420. DOI: 10.1038/s41586-019-1549-9.
-
Balmus G et al. (2019) ATM orchestrates the DNA-damage response to counter toxic non-homologous end-joining at broken replication forks. Nat Commun 10(1):87. DOI: 10.1038/s41467-018-07729-2.
Biography
Prof Steve Jackson PhD FRS FMedSci
Senior Group Leader at Cancer Research UK Cambridge Institute; Frederick James Quick Professor of Biology and Member of the Department of Biochemistry, University of Cambridge
My academic research aims to better understand how cells detect and repair DNA damage and signal its presence to affect myriad aspects of cell biology. My laboratory has a strong track record of using a broad range of cell and molecular biological techniques and approaches, in both mammalian and yeast cells.
My discovery that DNA breaks trigger DNA-PK kinase activity established a paradigm for DNA damage detection and signalling, leading us to identify the first non-homologous end-joining (NHEJ) DNA-repair proteins. Subsequently, we identified and/or determined functions for these and other core NHEJ proteins, showing how they and additional DDR factors interact in regulated ways.
We have defined many aspects of the DDR, particularly those controlling and/or mediated by phosphorylation, ubiquitylation, sumoylation and/or chromatin modifications. In 1995, we found that DDR-enzyme-targeting compounds could selectively kill cancer cells, and that defects in some cause DNA-damage hypersensitivity in the absence of others.
Knowing that DDR defects occur in cancer, I founded KuDOS Pharmaceuticals: the world’s first DNA-repair company. KuDOS developed the innovative precision anti-cancer medicine olaparib/LynparzaTM that is enhancing and extending the lives of thousands of patients worldwide. My academic laboratory is currently further defining mechanisms of DNA repair and associated processes, with a view to delivering new biological insights and identifying new therapeutic opportunities for cancer and other diseases.
It is my belief that a deeper knowledge of DNA repair and DDR pathways will yield better understandings of diseases that arise when such pathways are impaired or deregulated.
Notable achievements and honours
-
2023Knight Bachelor
-
2022Johann Anton Merck Award
-
2020Royal Society Mullard Award
-
2019Fondation ARC Léopold Griffuel Award for Translational and Clinical Research
-
2016Royal Netherlands Academy of Arts and Sciences Dr. A.H. Heineken Prize for Medicine
-
2016King Faisal International Prize for Science
-
2015Gagna A. & Ch. Van Heck Prize
-
2011Royal Society Buchanan Medal
-
2008Fellow of the Royal Society
-
2001Academy of Medical Sciences, UK