Emma Rawlins
Senior group leaderResearch summary
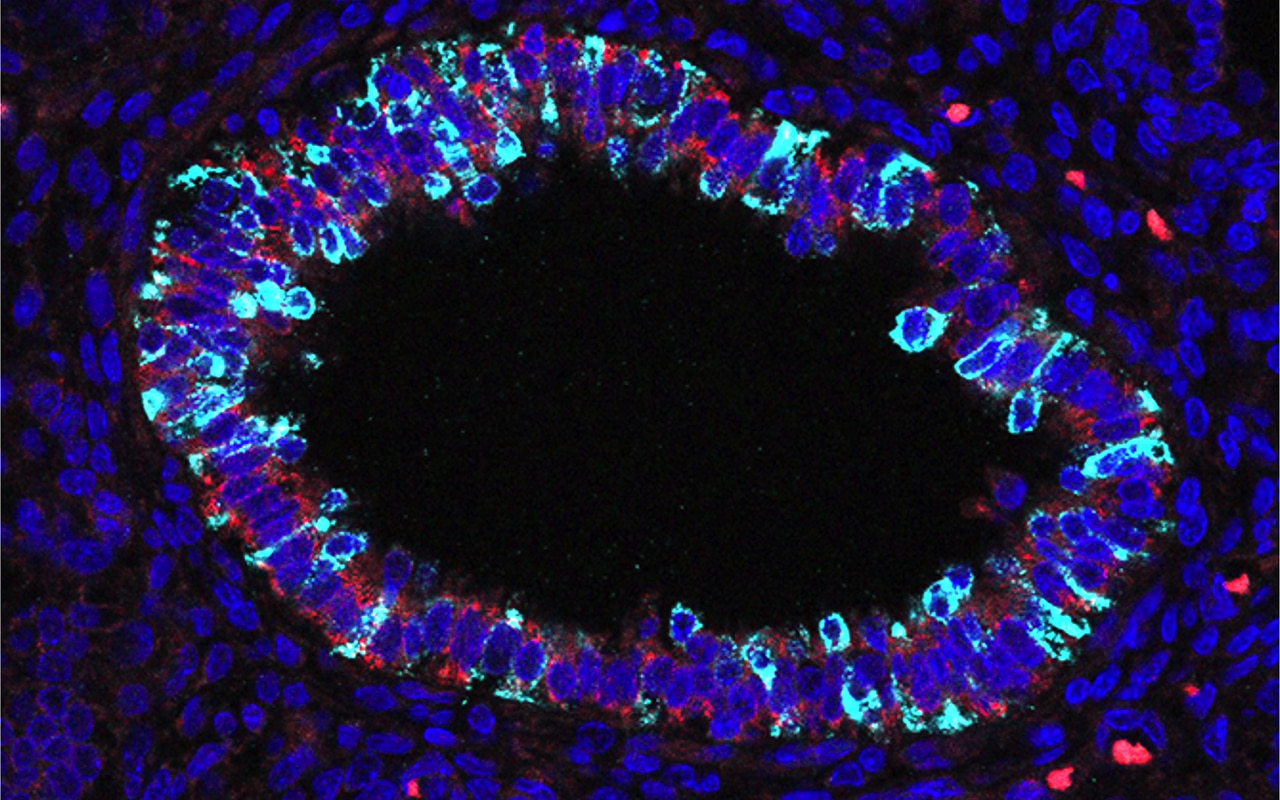
Stem and progenitor cells in the mammalian lung
How do stem cells build and maintain the lung? The complicated three-dimensional structure of our lungs is essential for respiration and host defence. Building this structure relies on the correct sequence of division and differentiation events by lung progenitor cells, which also maintain the slowly turning-over airway epithelium in the adult. How is the production of different cell types controlled in embryonic development and adult maintenance?
We apply mouse genetics, live imaging, single-cell molecular analysis and mathematical modelling to understand lung stem cells, with a longer-term aim of directing endogenous lung cells to repair, or regenerate, diseased tissue.

Selected publications
-
Lim K et al.(2023) Organoid modeling of human fetal lung alveolar development reveals mechanisms of cell fate patterning and neonatal respiratory disease. Cell Stem Cell 30, 1-18. Published online December 8, 2022. DOI: 10.1016/j.stem.2022.11.013.
-
He P et al. (2022) A human fetal lung cell atlas uncovers proximal-distal gradients of differentiation and key regulators of epithelial fates. Cell 185(25): 4841-4860.e25. DOI: 10.1016/j.cell.2022.11.005.
-
Sun D et al. (2022) SOX9 maintains human foetal lung tip progenitor state by enhancing WNT and RTK signalling. The EMBO Journal e111338. DOI: 10.15252/embj.2022111338.
-
Sun D et al. (2021) A functional genetic toolbox for human tissue-derived organoids. Elife 10: e67886 DOI: 10.7554/eLife.67886. DOI: 10.7554/eLife.67886.
-
Ziegler CGK et al., …HCA Lung Biological Network (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181(5):1016-1035.e19 DOI: 10.1016/j.cell.2020.04.035.
-
Nikolić M et al. (2017) Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife 6: e26575. DOI: 10.7554/eLife.26575.
Biography
Dr Emma Rawlins PhD
MRC Senior Non-Clinical Fellow, Member of the University Department of Physiology, Development and Neuroscience
Emma Rawlins obtained her PhD in developmental biology from the University of Edinburgh in 2002. She performed postdoctoral work with Prof Brigid Hogan at Duke University in 2004–2009 where she identified stem cell populations in the developing, homeostatic and repairing mouse lungs.
In 2009 she started her lab at the Gurdon Institute, University of Cambridge and in 2020 was promoted to senior Group Leader. Emma is a member of the Department of Physiology, Development and Neuroscience. Her lab works on lung stem and progenitor cell biology, combining innovative human organoid models with mouse genetics.
Notable achievements and honours
-
2022British Society for Developmental Biology Cheryll Tickle Award
-
2017MRC Senior Non-Clinical Fellowship
-
2012MRC Centenary Award
-
2011March of Dimes Basil O’Connor Award
-
2010EuroSyStem Innovative Project award
-
2009MRC Career Development Fellowship
-
2007Parker B Francis Postdoctoral Fellowship in Pulmonary Research
-
2002Wellcome Trust Prize Fellowship
Research group
-
Claire Bunn
PhD Student
-
Dr Livia Delpiano
Research Associate
-
Ziqi Dong
PhD Student
-
Dr Peng He
Visitor
-
Tessa Hughes
PhD Student
-
Dr Quitz Jeng
Research Associate
-
Yihong Li
Visiting Student
-
Odara Megadora
PhD Student
-
Dr John Russell
Research Associate
-
Vanesa Sokleva
PhD Student