Daniel St Johnston
Senior group leaderResearch summary
Polarising epithelial cells and body axes
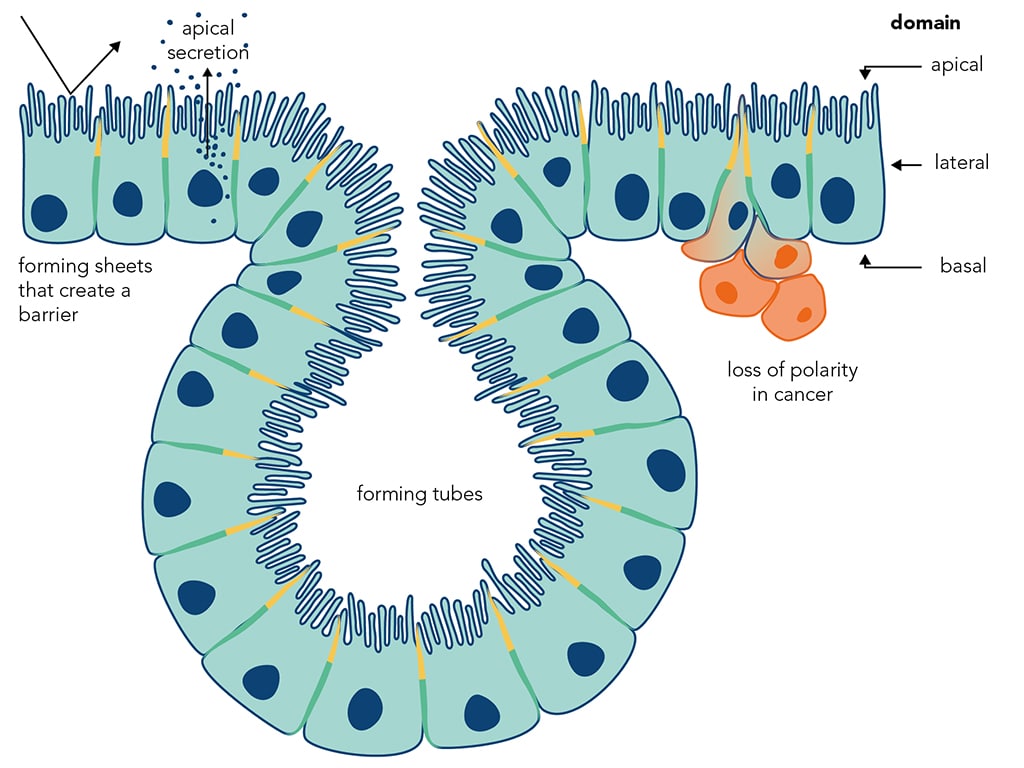
How do cells know ‘up’ from ‘down’? Most cells in the body perform different functions at opposite sides of the cell. This cell polarity is essential in development, for example: in determining the head-to-tail axis of many animals, for cell migration and for asymmetric stem-cell divisions. Furthermore, loss of polarity is a hallmark of tumour cells and is thought to contribute to tissue invasion and metastasis.
Our work focuses on epithelia, the sheets of polarised cells that form barriers between compartments and make up most of our organs and tissues. We study the factors that mark different sides of epithelial cells and how these organise the internal cell architecture, using the Drosophila intestine and the follicle cell epithelium as models.

Selected publications
-
Chen J and St Johnston D. (2022) De novo apical domain formation inside the Drosophila adult midgut epithelium. Elife 11, e76366. DOI: 10.7554/elife.76366.
-
Buckley CE and St Johnston D. (2022) Apical–basal polarity and the control of epithelial form and function. Nat Rev Mol Cell Bio, 1–19. DOI: 10.1038/s41580-022-00465-y.
-
Doerflinger H et al. (2021) The Drosophila anterior-posterior axis is polarized by asymmetric myosin activation. Current Biology 32(2): 374-385.e4. DOI: 10.1016/j.cub.2021.11.024.
-
Nashchekin D et al. (2021) Symmetry breaking in the female germline cyst. Science 374 (6569): 874-879. DOI: 10.1126/science.abj3125.
-
Fic W et al. (2021) RhoGAP19D inhibits Cdc42 laterally to control epithelial cell shape and prevent invasion. J Cell Biol, 220 (4): e202009116. DOI: 10.1083/jcb.202009116.
-
Lovegrove H et al. (2019) The role of integrins in Drosophila egg chamber morphogenesis. Development 146: dev182774. DOI: 10.1242/dev.182774.
-
Erdmann RS et al. (2019) Labeling Strategies Matter for Super-Resolution Microscopy: A Comparison between HaloTags and SNAP-tags. Cell Chem Biol 26(4):584-592.e6. DOI: 10.1016/j.chembiol.2019.01.003.
-
Fic W et al. (2019) Drosophila IMP regulates Kuzbanian to control the timing of Notch signalling in the follicle cells. Development 146: dev168963. DOI: 10.1242/dev.168963.
-
Chen J et al. (2018) An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol 16: e3000041. DOI: 10.1371/journal.pbio.3000041.
Biography
Prof Daniel St Johnston PhD FRS FMedSci
Wellcome Principal Research Fellow, Professor of Developmental Genetics, Member of the University Department of Genetics
Research group
-
Dr Edward Allgeyer
Senior Research Associate
-
Dr Jia Chen
Research Associate
-
Dr Helene Doerflinger
Research Associate/ Public Engagement Manager
-
Dr Xiaoming Fang
Research Associate
-
Xiao Li He
Senior Research Laboratory Technician
-
Dr Dmitry Nashchekin
Research Associate
-
John Overton
Senior Research Lab Technician
-
Dr Jenny Richens
Research Associate
-
Samarpita Sen
PhD Student
-
Dr George Sirinakis
Senior Research Associate
-
Dr Mihoko Tame
Research Associate
-
Dr Joseph Jose Thottachery
Research Associate
-
Xixi Zhu
PhD Student