David Fernandez-Antoran
Group leaderResearch summary
Radiation biology and cell competition
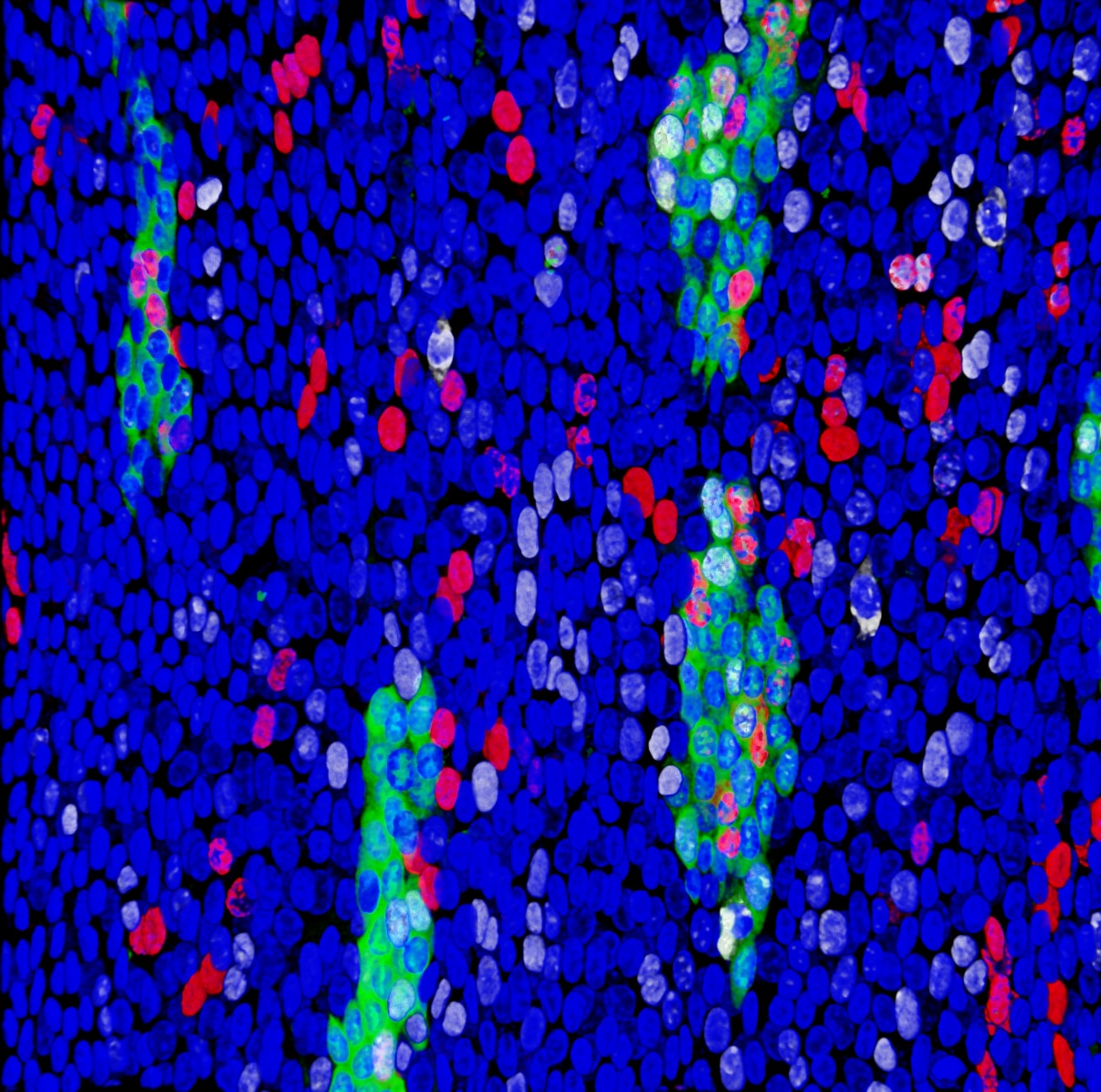
Healthy adult epithelial tissues progressively accumulate clones of cells carrying mutations implicated in cancer and their expansion follows Darwinian evolution rules. Some mutations can increase cell fitness and promote the growth of clones at the expenses of the non-mutated normal adjacent cells, in a process of clonal competition.
Ionising radiation has long been studied as one of the most common environmental mutagenic agents that promotes tumour formation by damaging DNA and creating new oncogenic mutations. However, little is known about its effects on cell competition, clonal evolution and tissue dynamics.
Email: d.fernandez-antoran [at] gurdon [dot] cam [dot] ac [dot] uk.

Selected publications
-
Hoo R, Ruiz-Morales ER, Kelava I et al. (2024) Acute response to pathogens in the early human placenta at single-cell resolution. Cell Syst. May 15;15(5):425-444.e9. DOI: 10.1016/j.cels
-
Murai K et al. (2022) p53 mutation in normal esophagus promotes multiple stages in carcinogenesis but is constrained by clonal competition. Nature Communications Oct 20;13(1):6206. DOI: 10.1038/s41467-022-33945-y
-
Colom B et al. (2021) Mutant clones in normal epithelium outcompete and eliminate emerging tumours. Nature 598: 510–514. DOI: 10.1038/s41586-021-03965-7.
-
Fowler JC et al. (2021) Selection of Oncogenic Mutant Clones in Normal Human Skin Varies with Body Site. Cancer Discovery DOI: 10.1158/2159-8290.CD-20-1092.
-
Piedrafita G et al. (2020) A single-progenitor model as the unifying paradigm of epidermal and esophageal epithelial maintenance in mice. Nature Communications 11: 1429. DOI: 10.1038/s41467-020-15258-0.
-
Fernandez-Antoran D et al. (2019) Outcompeting p53-mutant cells in the normal esophagus by redox manipulation. Cell Stem Cell 25: P329-341.E6. DOI: 10.1016/j.stem.2019.06.011.
-
Martincorena I et al. (2018) Somatic mutant clones colonize the human esophagus with age. Science 362: 911-917. DOI: 10.1126/science.aau3879
Biography
David Fernandez-Antoran PhD, CRUK-RadNet Group Leader, Member of the University Department of Pathology
I did my PhD in Immunology at the Spanish National Centre for Biotechnology (CNB-Madrid), where I studied the role of the proto-oncogene c-myc and its partner max in the development and maturation of B-lymphocytes in vivo.
In 2013 I started a postdoctoral position in the laboratory of Prof. Philip H. Jones (MRC-Cancer Unit / University of Cambridge and The Sanger Institute), funded by European Radiation Agency, where I developed a profound knowledge of epithelial tissue architecture, cell behaviour, radiation biology and cancer development. I studied how epithelial progenitor cells respond to environmental factors like low doses of ionising radiation and how this affects clonal competition within healthy tissues. I demonstrated that these exposures promote the expansion of oncogenic mutations in the oesophageal epithelium. I also demonstrated for the first time how to interfere with this process and deplete mutant cells by manipulating redox status, which opens a new field of study for future interventions in humans in order to reduce cancer risk.
In collaboration with colleagues from the Wellcome Sanger Institute and Wellcome-MRC Cambridge Stem Cell Institute, I developed and optimized a new model of in vitro long-term and self-maintaining 3D primary culture using mouse and human epithelial tissues. This system affords a novel concept in the field and allows long-term studies on tissue regeneration, cell-to-cell dynamic interactions, drug responses as well as monitoring early steps of tumour development in vitro.
I started my own laboratory, funded by CRUK-RadNet, at The Gurdon Institute in 2020. Our aim is to understand how radiotherapy treatments (among other environmental factors), modulate cell competition mechanisms in different epithelia, by introducing new selective pressures that change the mutational landscape and might affect tissue function and promote carcinogenesis.
Notable achievements and honours
-
2024Scientific Deputy Director of CRUK-RadNet Cambridge
-
2022CRUK–RadNet Seed funding for FLASH radiotherapy studies
-
2022NC3Rs–CRUK Skills and Knowledge Transfer grant
-
2022Co-Chair CRUK–RadNet Emerging Radiotherapies Technologies
-
2022CRUK–RadNet Infrastructure grant
-
2021Isaac Newton Trust / Wellcome Trust ISSF / University of Cambridge Joint Research Grants Scheme
-
2020CRUK–RadNet Grant
-
2013RISK - IR GRANT. FP7-EURATOM-FISSION
Research group
-
Virgile Caumont
Visiting Student
-
Ines Ferreira
PhD Student
-
Kasandra Malasi
Senior Research Assistant
-
Dr Alberto Pradilla-Dieste
Research Associate
-
Fillipa Samella
Part II Student
-
Dr Jose Valverde Lopez
Research Associate
-
Dr Eirini Terpsi Vitti
Research Associate