Identifying the molecular machinery for mitochondrial genome maintenance
November 10, 2022
Read more
Tissue Force Microscopy (TiFM) is a highly sensitive, minimally invasive tool which can quantitively characterise forces within the developing chicken embryo. This method can also be used to apply stable and physiologically relevant loads to drive tissue deformation followed by characterisation of the tissue responses.
Chan CU, Xiong F, Michaut A, Vidigueira JMN, Pourquié O, Mahadevan L. Direct force measurement and loading on developing tissues in intact avian embryos. Development. 2023 May 1;150(9):dev201054. DOI: 10.1242/dev.201054.
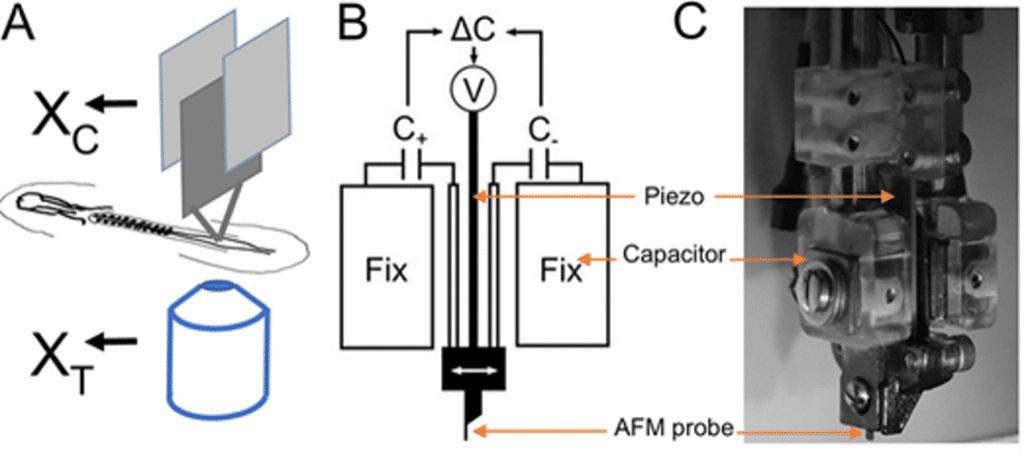
(A) The concept of TiFM. The design takes advantage of the flatness of the early avian embryo. XC is the holder ‘chip’ position measured by the capacitors, XT is the probe ‘tip’ position measured by the microscope. The difference between them is a measure of the deflection of the cantilever beam. (B,C) Probe holder and capacitors. Two capacitor plates and the piezo are integrated for position control and measurement against two fixed capacitor plates. C, capacitance; V, voltage. (C) A side photo of the assembled probe holder.
Developmental morphogenesis is driven by tissue stresses acting on tissue rheology. Direct measurements of forces in small tissues (100 µm-1 mm) in situ, such as in early embryos, require high spatial precision and minimal invasiveness. Here, we introduce a control-based approach, tissue force microscopy (TiFM), that integrates a mechanical cantilever probe and live imaging with closed-loop feedback control of mechanical loading in early chicken embryos. By testing previously qualitatively characterized force-producing tissues in the elongating body axis, we show that TiFM quantitatively captures stress dynamics with high sensitivity. TiFM also provides the means to apply stable, minimally invasive and physiologically relevant loads to drive tissue deformation and to follow the resulting morphogenetic progression associated with large-scale cell movements. Together, TiFM allows us to control tissue force measurement and manipulation in small developing embryos, and promises to contribute to the quantitative understanding of complex multi-tissue mechanics during development.