Advances in the in vitro derivation of human gametes bring hopes, but raises questions
November 14, 2023
Read more
The Simons lab collaborated with the Hospital for Sick Children and other researchers in Toronto to examine the cellular lineages involved in adult and paediatric cerebral tumours. First generating an atlas of mouse cerebral development, from single-cell transcriptomics on 100,000 cells, they compared this with single-cell data from human glial tumours to show that tumour cells show distinct development-like signatures.
In addition, a validation experiment showed that transformation of embryonic cerebral precursor cells using a transgenic mouse model gave rise to cellularly heterogeneous adult cerebral tumours that show an embryonic/juvenile identity.
As well as providing molecular targets for early detection, the association of tumour growth with the activation of developmental programmes may suggest intervention strategies that target differentiation rather than cell proliferation. It also should lead to a greater precision of targeting the heterogeneous cell types that comprise specific childhood and adult cerebral tumours.
Hamed AA et al. (2022) A brain precursor atlas reveals the acquisition of developmental-like states in adult cerebral tumours. Nature Communications 13: 4178. DOI: 10.1038/s41467-022-31408-y.
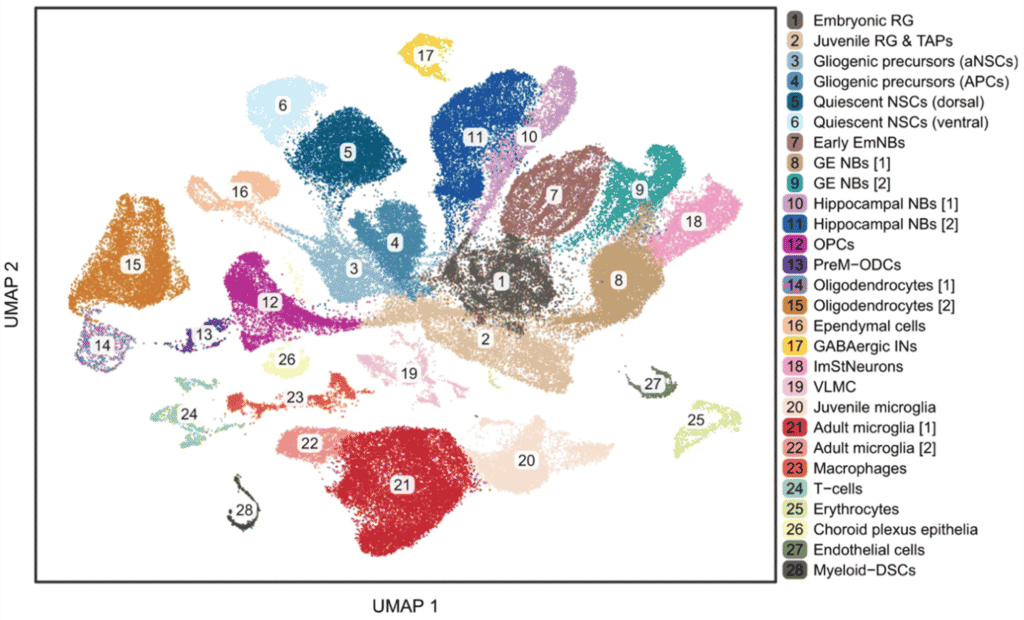
Fig 1b from the paper: Plot of >100,000 cells in developing mouse brain identifying 28 transcriptionally and biologically distinct cell types.
Human cerebral cancers are known to contain cell types resembling the varying stages of neural development. However, the basis of this association remains unclear.
Here, we map the development of mouse cerebrum across the developmental time-course, from embryonic day 12.5 to postnatal day 365, performing single-cell transcriptomics on >100,000 cells. By comparing this reference atlas to single-cell data from >100 glial tumours of the adult and paediatric human cerebrum, we find that tumour cells have an expression signature that overlaps with temporally restricted, embryonic radial glial precursors (RGPs) and their immediate sublineages.
Further, we demonstrate that prenatal transformation of RGPs in a genetic mouse model gives rise to adult cerebral tumours that show an embryonic/juvenile RGP identity.
Together, these findings implicate the acquisition of embryonic-like states in the genesis of adult glioma, providing insight into the origins of human glioma, and identifying specific developmental cell types for therapeutic targeting.