Untangling the mechanism of human primordial germ cell specification
April 11, 2022
Read more
The study was led by Dr Xufeng Xue and Prof Jianping Fu, University of Michigan. Freddy Wong from the Surani lab, now at the Sanger Institute, contributed to early discussions on how the rostral-caudal neural tube (NT) is descended from two different types of cells, with the caudal NT contributed mainly from neuromesodermal progenitors (NMP). Until now, organoids would only incorporate either rostral or caudal NT identities. For the first time, a system incorporating both in a continuous luminal structure allows possible short or long range communication between rostral-caudal identities that can be important in regulating their strict regionalisation and coordinated development on a tissue level. These structures also have the potential to model the neuronal establishment and signalling between these two NT compartments.
Xue X et al. A Patterned Human Neural Tube Model Using Microfluidic Gradients. Nature 2024 Apr;628(8007):391-399. DOI: 10.1038/s41586-024-07204-7.
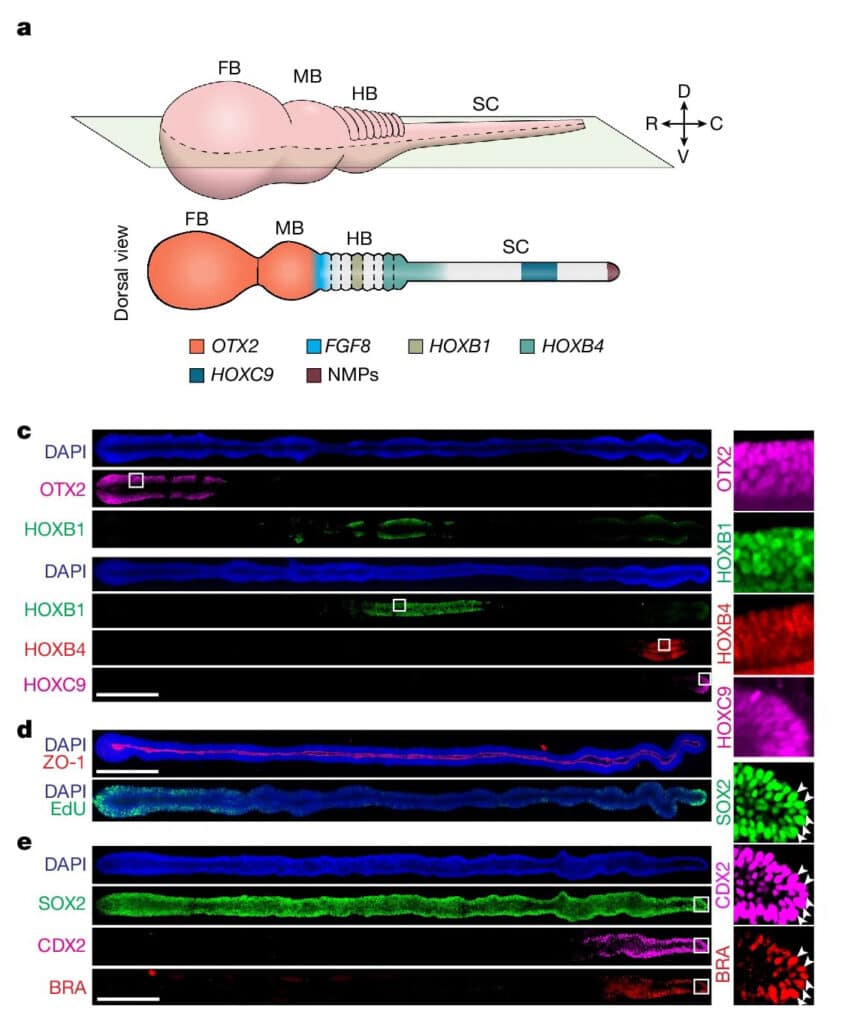
Fig. 1 (cropped) a, A 3D and dorsal view of the human NT in vivo patterned along the R–C axis into the FB, MB, HB and SC. NMPs develop at the SC caudal end. Regionalization of the NT leads to patterned gene expression, with upregulated OTX2 expression in the FB and MB, FGF8 at the MB–HB boundary and HOX family genes in the HB and SC as indicated. c, Left, stitched confocal micrographs showing R–C patterned µNTLS on day 7 immunostained for OTX2, HOXB1, HOXB4 and HOXC9 as indicated. Right, zoom-in views of the boxed regions as indicated. d, Stitched confocal micrographs showing R–C patterned µNTLS on day 7, with ZO-1 and EdU staining. e, Left, stitched confocal micrographs showing R–C patterned µNTLS on day 4 immunostained for SOX2, CDX2 and BRA. Right, zoom-in views of boxed regions as indicated. SOX2+CDX2+BRA+ cells are marked by arrowheads.
Human nervous system is arguably the most complex but highly organized organ. Foundation of its complexity and organization is laid down during regional patterning of neural tube (NT), the embryonic precursor to human nervous system. Historically, studies of NT patterning have relied on animal models to uncover underlying principles. Recently, human pluripotent stem cell (hPSC)-based models of neurodevelopment, including neural organoids1-5 and bioengineered NT development models6-10, are emerging. However, existing hPSC-based models fail to recapitulate neural patterning along both rostral-caudal (R–C) and dorsal-ventral (D–V) axes in a three-dimensional (3D) tubular geometry, a hallmark of NT development. Herein we report a hPSC-based, microfluidic NT-like structure (or μNTLS), whose development recapitulates some critical aspects of neural patterning in both brain and spinal cord (SC) regions and along both R–C and D–V axes. The μNTLS was utilized for studying neuronal lineage development, revealing prepatterning of axial identities of neural crest (NC) progenitors and functional roles of neuromesodermal progenitors (NMPs) and caudal gene CDX2 in SC and trunk NC development. We further developed D–V patterned, microfluidic forebrain-like structures (μFBLS) with spatially segregated dorsal and ventral regions and layered apicobasal cellular organizations that mimic human forebrain pallium and subpallium developments, respectively. Together, both μNTLS and μFBLS offer 3D lumenal tissue architectures with an in vivo-like spatiotemporal cell differentiation and organization, promising for studying human neurodevelopment and disease.