DREAM functions are mediated by THAP proteins
October 19, 2021
Read more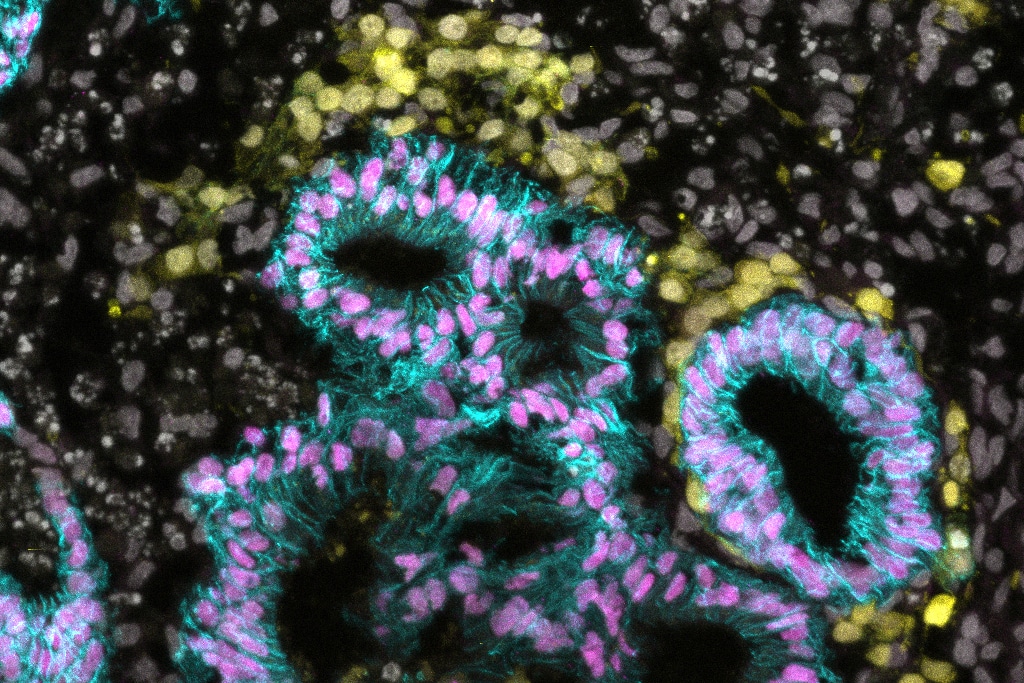
A detailed protocol from the Surani lab developed by Alves-Lopes and Wong describes the specification of human primordial germ cell-like cells from precursors transitioning between primed and naïve pluripotency. These human primordial germ cell-like cells harbour an enhanced progression capability, which is supported by human hindgut organoids. These experimental approaches can pave the way to uncover the principles of the crosstalk between human primordial germ cells as they migrate through the hindgut towards the developing gonads.
Alves-Lopes JP, Wong FCK, Surani A (2024). Human primordial germ cell-like cells specified from resetting precursors develop in human hindgut organoids. Nature Protocols (2024) DOI: 10.1038/s41596-023-00945-1.
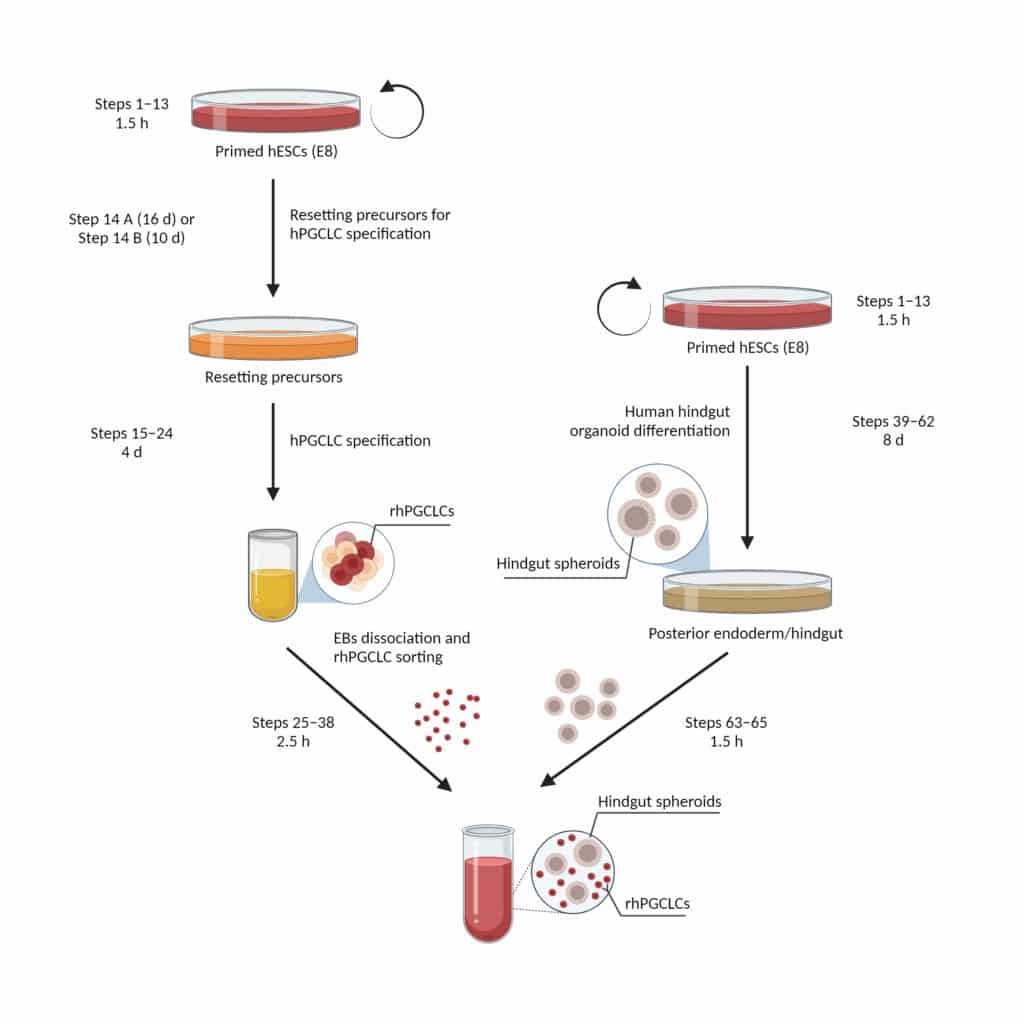
Human primordial germ cells (hPGCs), the precursors of eggs and sperm, start their complex development shortly after specification and during their migration to the primitive gonads. Here, we describe protocols for specifying hPGC-like cells (hPGCLCs) from resetting precursors and progressing them with the support of human hindgut organoids. Resetting hPGCLCs (rhPGCLCs) are specified from human embryonic stem cells (hESCs) transitioning from the primed into the naïve state of pluripotency. Hindgut organoids are also derived from hESCs following a sequential differentiation into posterior endoderm/hindgut fate. Both rhPGCLCs and hindgut organoids are combined and co-cultured for 25 days. The entire procedure takes approximately 1.5 months and can be successfully implemented by a doctoral or graduate student with basic skills and experience in hESC cultures. The co-culture system supports the progression of rhPGCLCs at a developmental timing analogous to that observed in vivo. Compared with previously developed hPGCLC progression protocols, which depend on co-cultures with mouse embryonic gonadal tissue, our co-culture system represents a developmentally relevant model closer to the environment that hPGCs first encounter after specification. Together with the potential for investigations of events during hPGC specification and early development, these protocols provide a practical approach to designing efficient models for in vitro gametogenesis. Notably, the rhPGCLC-hindgut co-culture system can also be adapted to study failings in hPGC migration, which are associated with the aetiology of some forms of infertility and germ cell tumours.