Human primordial germ cell-like cells develop in human hindgut organoids
February 1, 2024
Read more
Exploring the mechanism of action of DREAM in C. elegans, the Ahringer lab uncovered two THAP domain proteins that co-localise with DREAM and function by different mechanisms to repress distinct gene targets. LIN-36 represses classical cell-cycle targets while LIN-15B represses germline-specific targets in the soma, and both differentially regulate DREAM binding. The authors propose that THAP domain proteins are key mediators of DREAM function.
Gal C et al. (2021) DREAM represses distinct targets by cooperating with different THAP domain proteins. Cell Reports 37(3): 109835. DOI: 10.1016/j.celrep.2021.109835
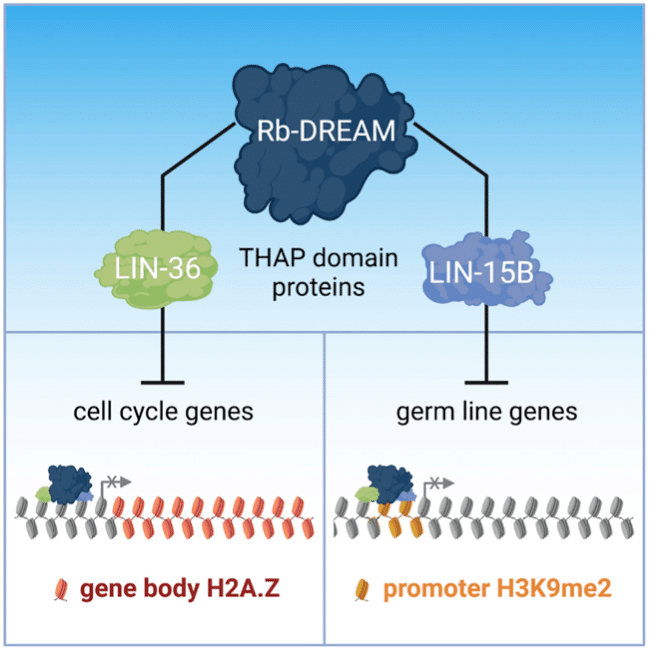
The DREAM (dimerization partner [DP], retinoblastoma [Rb]-like, E2F, and MuvB) complex controls cellular quiescence by repressing cell-cycle and other genes, but its mechanism of action is unclear.
Here, we demonstrate that two C. elegans THAP domain proteins, LIN-15B and LIN-36, co-localize with DREAM and function by different mechanisms for repression of distinct sets of targets. LIN-36 represses classical cell-cycle targets by promoting DREAM binding and gene body enrichment of H2A.Z, and we find that DREAM subunit EFL-1/E2F is specific for LIN-36 targets. In contrast, LIN-15B represses germline-specific targets in the soma by facilitating H3K9me2 promoter marking.
We further find that LIN-36 and LIN-15B differently regulate DREAM binding. In humans, THAP proteins have been implicated in cell-cycle regulation by poorly understood mechanisms. We propose that THAP domain proteins are key mediators of Rb/DREAM function.