Human fetal lung cell atlas released
December 8, 2022
Read more
The authors coined the term ‘mitoballs’ to describe this new cellular structure. Mitoballs, which are composed of hundreds of unfused mitochondria and some other organelles, assemble and dissemble in spermatocytes before the onset of meiosis. The team then identified Milton, an important motor adaptor regulating mitochondrial trafficking, to be crucial for mitoball formation. The loss of mitoballs in milton mutants is associated with abnormally swollen mitochondria and reduced male fertility. This work offers unparalleled insights into the dynamic nature of mitochondria and their remarkable ability to adapt to developmental needs. The findings also provide the opportunity to discover new targets for genetically engineering male sterility in pests and disease vectors, such as malaria-carrying mosquitos.
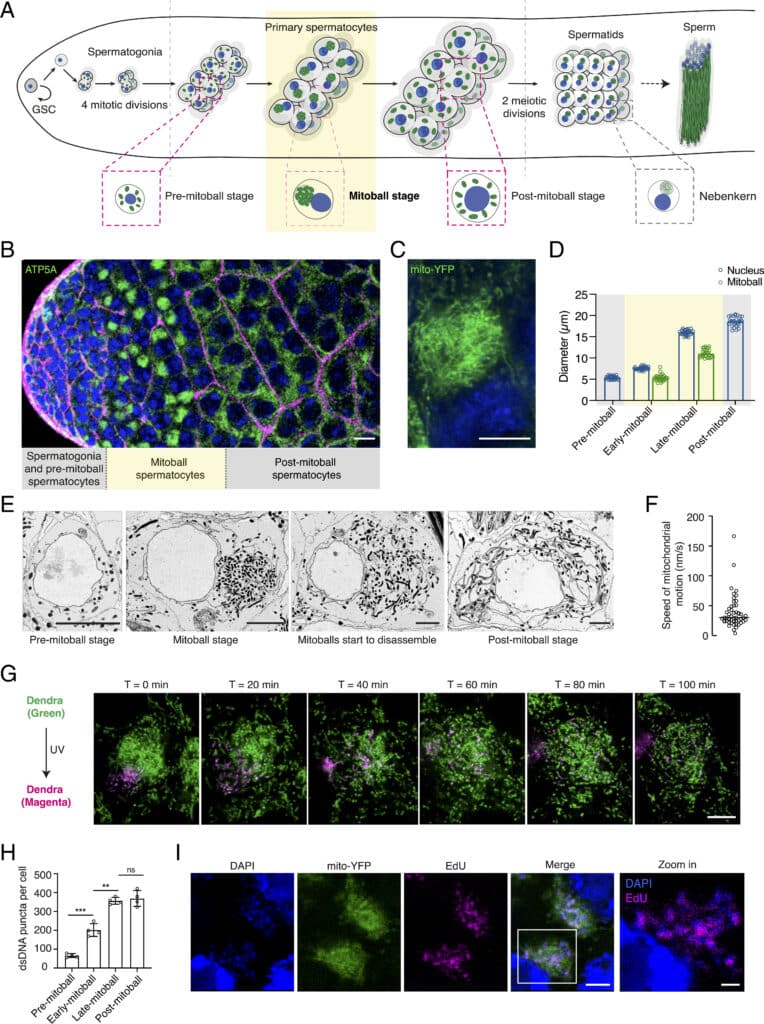
Mitochondria are dynamic organelles that undergo frequent remodelling to accommodate developmental needs. Here, we describe a striking organisation of mitochondria into a large ball-like structure adjacent to the nucleus in pre-meiotic Drosophila melanogaster spermatocytes, which we term ‘mitoball’.
Mitoballs are transient structures that colocalise with the endoplasmic reticulum, Golgi bodies, and the fusome. We observed similar pre-meiotic mitochondrial clusters in a wide range of insect species, including mosquitos and cockroaches. Through a genetic screen, we identified that Milton, an adaptor protein that links mitochondria to microtubule-based motors, mediates mitoball formation.
Flies lacking a 54 amino-acid region in the C terminus of Milton completely lacked mitoballs, had swollen mitochondria in their spermatocytes and showed reduced male fertility. We suggest that the pre-meiotic mitochondrial clustering is a conserved feature of insect spermatogenesis that supports sperm development.