Polarity axes in Drosophila
December 1, 2021
Read more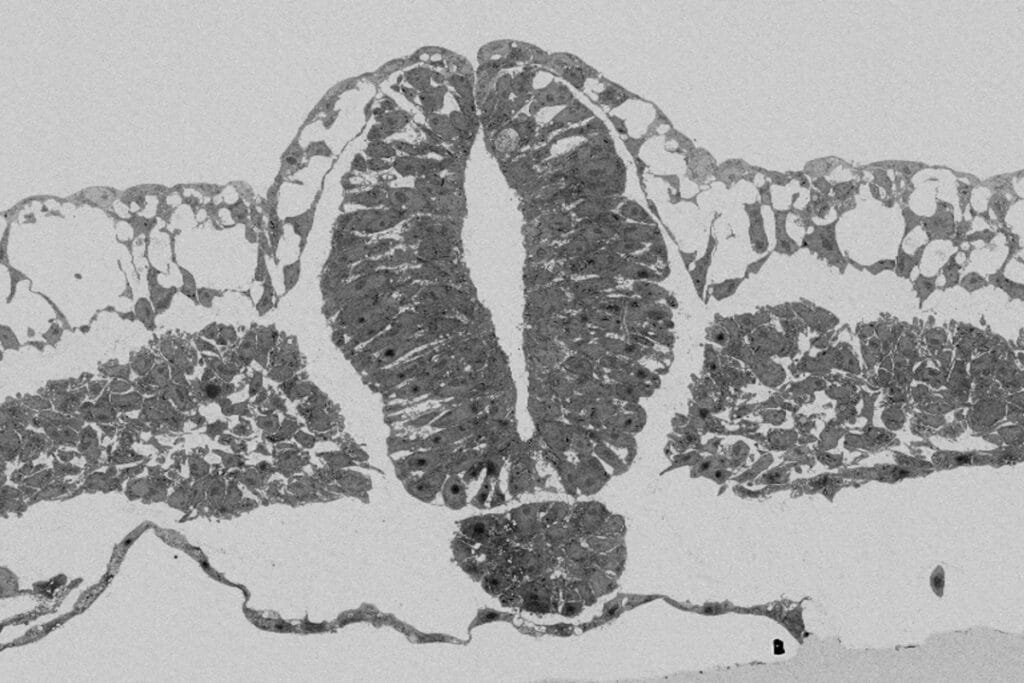
Using a combination of mechanical tools, the physical properties of the vitelline membrane were studied. This membrane provides key support to the developing avian and reptilian embryos. It was found that the early chicken embryo requires a reducing tension from the vitelline membrane to develop properly. This study provides an example of extraembryonic regulation of development via tissue forces.
Kunz, D, Wang A, Chan CU et al. Downregulation of extraembryonic tension controls body axis formation in avian embryos. Nature Communications 14, 3266 (2023). DOI: 10.1038/s41467-023-38988-3.
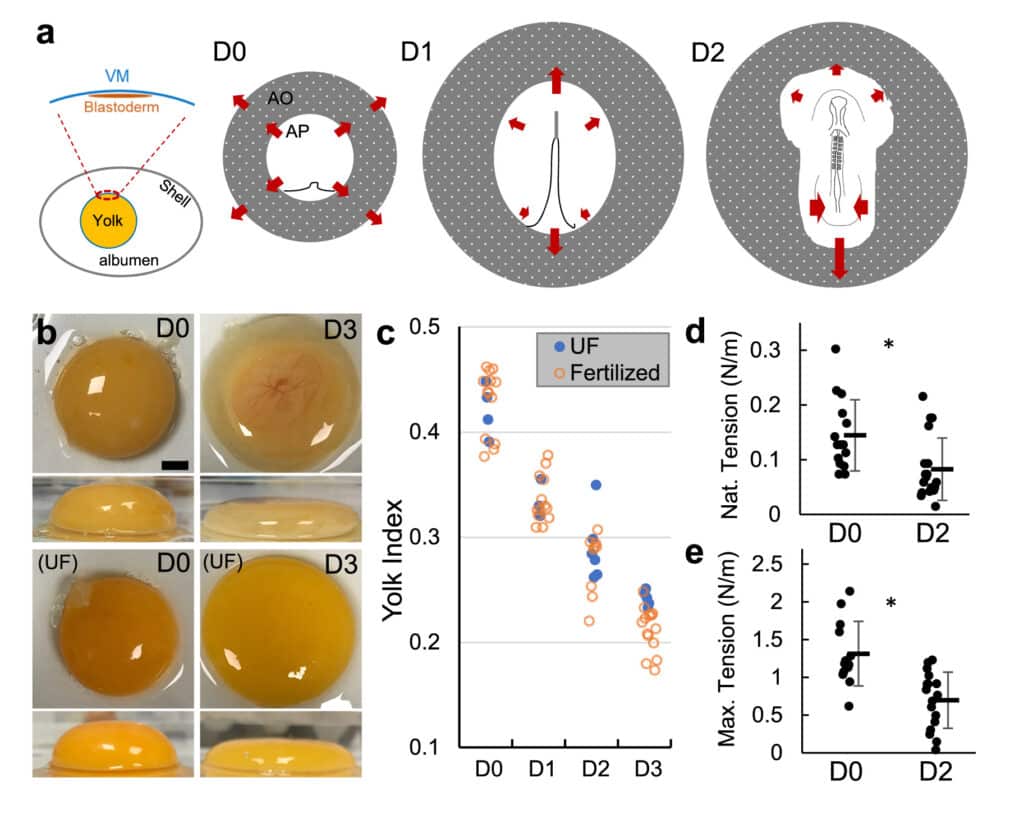
Fig. 1 | VM tension and strength decrease during early stages of development. a Diagram of early embryo tissue flow. Left panel shows the blastoderm in relation to the vitelline membrane (VM) and the egg. Right panels illustrate the early shape changes of the blastoderm including area opaca (AO) and area pellucida (AP). Each illustration (anterior to the top) shows the configuration of the blastoderm at approximately the beginning of the labeled incubation day (D0, D1 and D2). D0 involves blastoderm expansion and extension of the primitive streak. D1 and D2 involve continued regression of the streak, tissue convergence and body axis elongation that progress in an anterior to posterior order. Arrows indicate direction of tissue flow. b Yolk deformation on a flat surface at different stages (repre- sentative samples). Top and side views. UF unfertilized. Embryo is visible in the D3 image through the red-colored vasculature on the top center of the yolk. Scale bar: 1 mm. c Yolk index (height/diameter) over time. There is no significant difference between UF (n = 18) and fertilized (n = 50) in D0-D2 (p = 0.65 [D0], 0.96 [D1], 0.42 [D2], and 0.008 [D3], 2 tailed t-tests). d, e Native and maximum tensions sustained bytheVM.Barsindicatemean+/−SD.D0,n=15;D2,n=19.*p=0.006andp=2e-4, 2 tailed t-tests. ~20% of D2 measurements were on VMs taken from unfertilized eggs.
Embryonic tissues undergoing shape change draw mechanical input from extraembryonic substrates. In avian eggs, the early blastoderm disk is under the tension of the vitelline membrane (VM). Here we report that the chicken VM characteristically downregulates tension and stiffness to facilitate stage- specific embryo morphogenesis. Experimental relaxation of the VM early in development impairs blastoderm expansion, while maintaining VM tension in later stages resists the convergence of the posterior body causing stalled elongation, failure of neural tube closure, and axis rupture. Biochemical and structural analysis shows that VM weakening is associated with the reduction of outer-layer glycoprotein fibers, which is caused by an increasing albumen pH due to CO2 release from the egg. Our results identify a previously unrec- ognized potential cause of body axis defects through mis-regulation of extraembryonic tissue tension.